
#WHAT IS GAMP 5 GUIDELINES SERIES#
Security of this document is initially by physical access and then by a series of cascading passwords. It really depends on your company practices and procedure (which also must be documented and approved) as to whether the GAMP 5 doctrine is in electronic format with or without, e signatures. The original SOP once approved will reside in a safe and secure location in change control (this is a regularly audited requirement). In the majority of cases this requirement is fulfilled by using a standard operating practice documents (SOP’s) to encapsulate the GAMP 5 methodology chosen.

The regulators mandate that all good automated manufacturing processes and methods used to produce a regulated product must be documented and be company approved. It should become a master reference document and its use will ensure that all your company regulatory activities will be compliant with the appropriate regulations. On the other hand the work put in to developing your own RPP pays endless dividends, since it will document the responsibilities of all personnel, along with defining the scope of all company qualification processes. The GAMP 5 committee has tried to be all things to all people, and in the opinion of many fail to be specific enough to be used as anything other than a discussion document.
#WHAT IS GAMP 5 GUIDELINES MANUAL#
This manual should then be the definitive authority on all Validation Online company matters. GAMP 5 compliance documents should be closely studied and the requirements, as applicable to your company’s activities, should be extrapolated and developed into your quality policies and procedures manual.
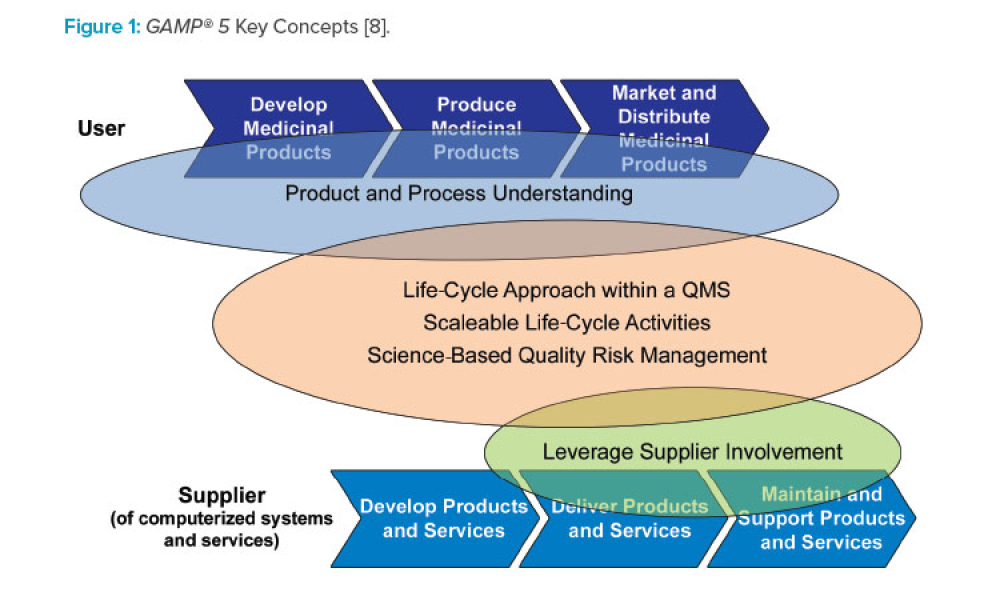
So there is often contentious material, statements and or methods included. Only difference is it will have titled them, Company Regulatory Practices and Procedures (CRPP) (or something similar), and they will be specific to the company.Īll the GAMP 5 guidance documents have been authored by committees, and not everybody in a committee can get their own say. However the reality of the situation is that our regulatory requirements for cGMP are published in 21 CFR Part 820/210/211/11 and other government issued edicts.Ī company compliant with its regulatory requirements will have written its own equivalent of the GAMP-1/2/3/4/5 series. Although GAMP 5 has no legal standing and is purely advisory, it does contain information and methodologies that are of interest to anyone engaged in validation activities within the cGMP regulated environment. The acronym GAMP-5 refers to "Good Automatic Manufacturing Practices issue 5", document.


 0 kommentar(er)
0 kommentar(er)
